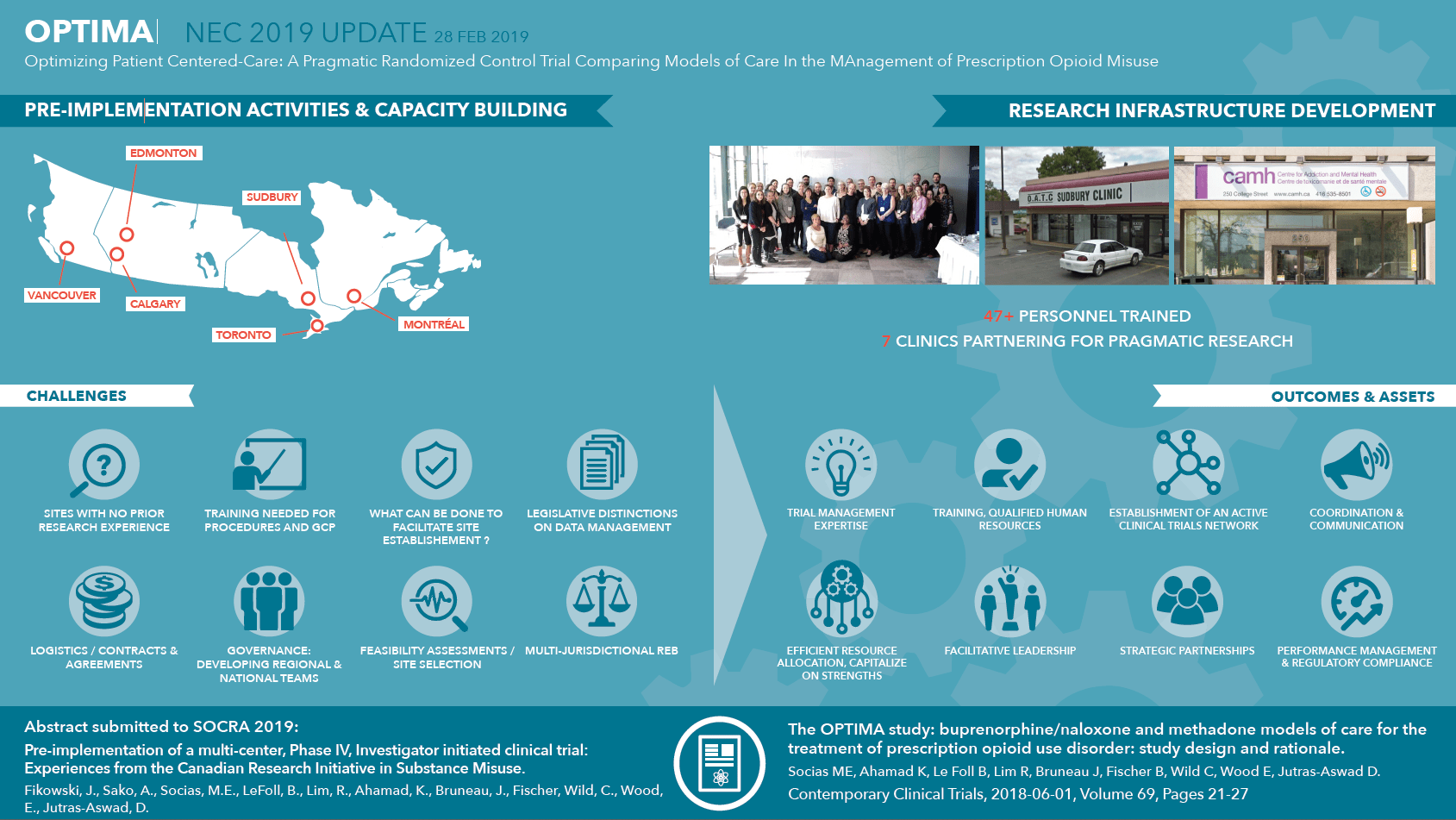
- Dissemination of results in progress
Context
Prevention and treatment of opioid use disorder (OUD) has become an urgent public health priority in Canada.
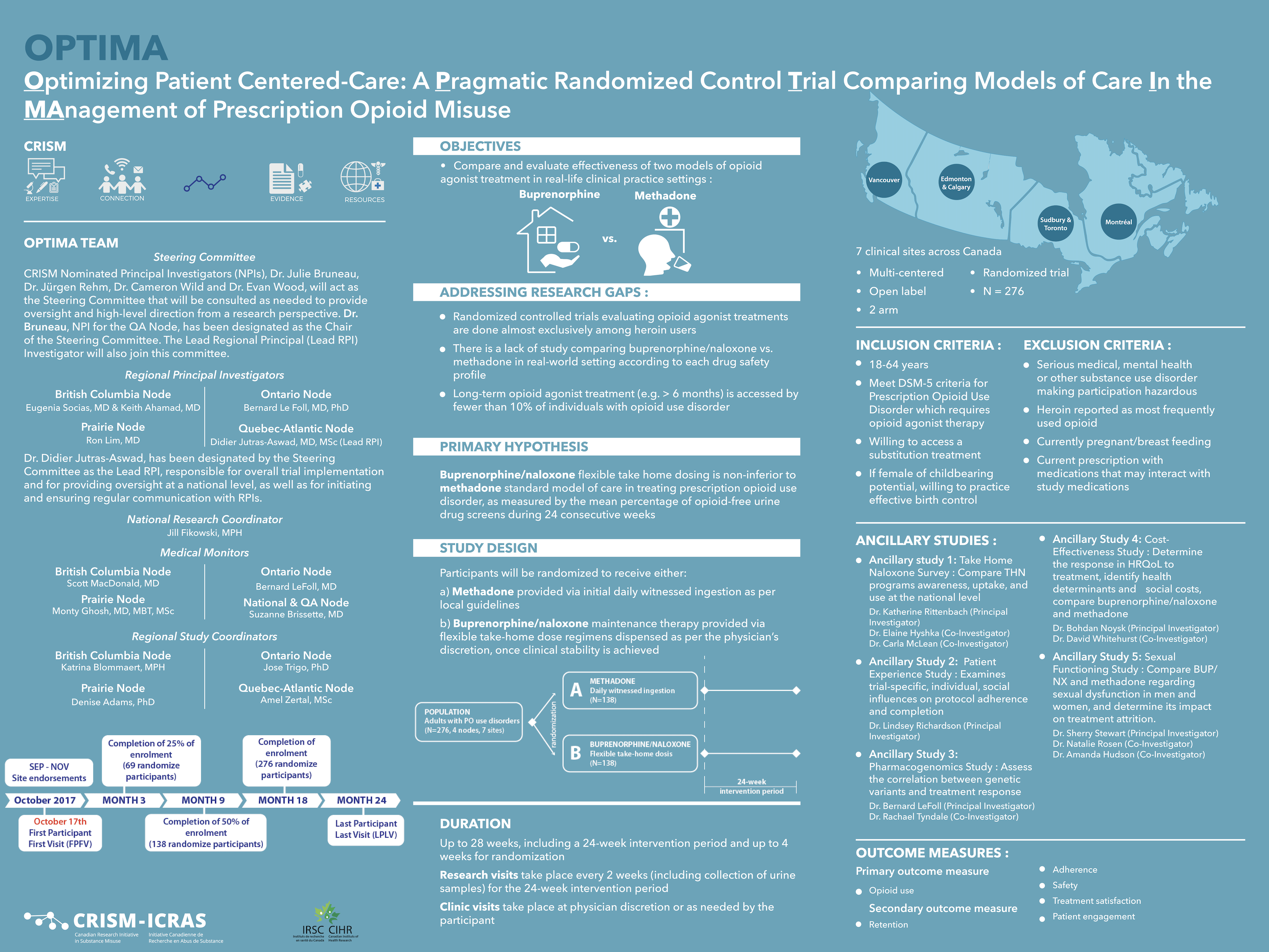
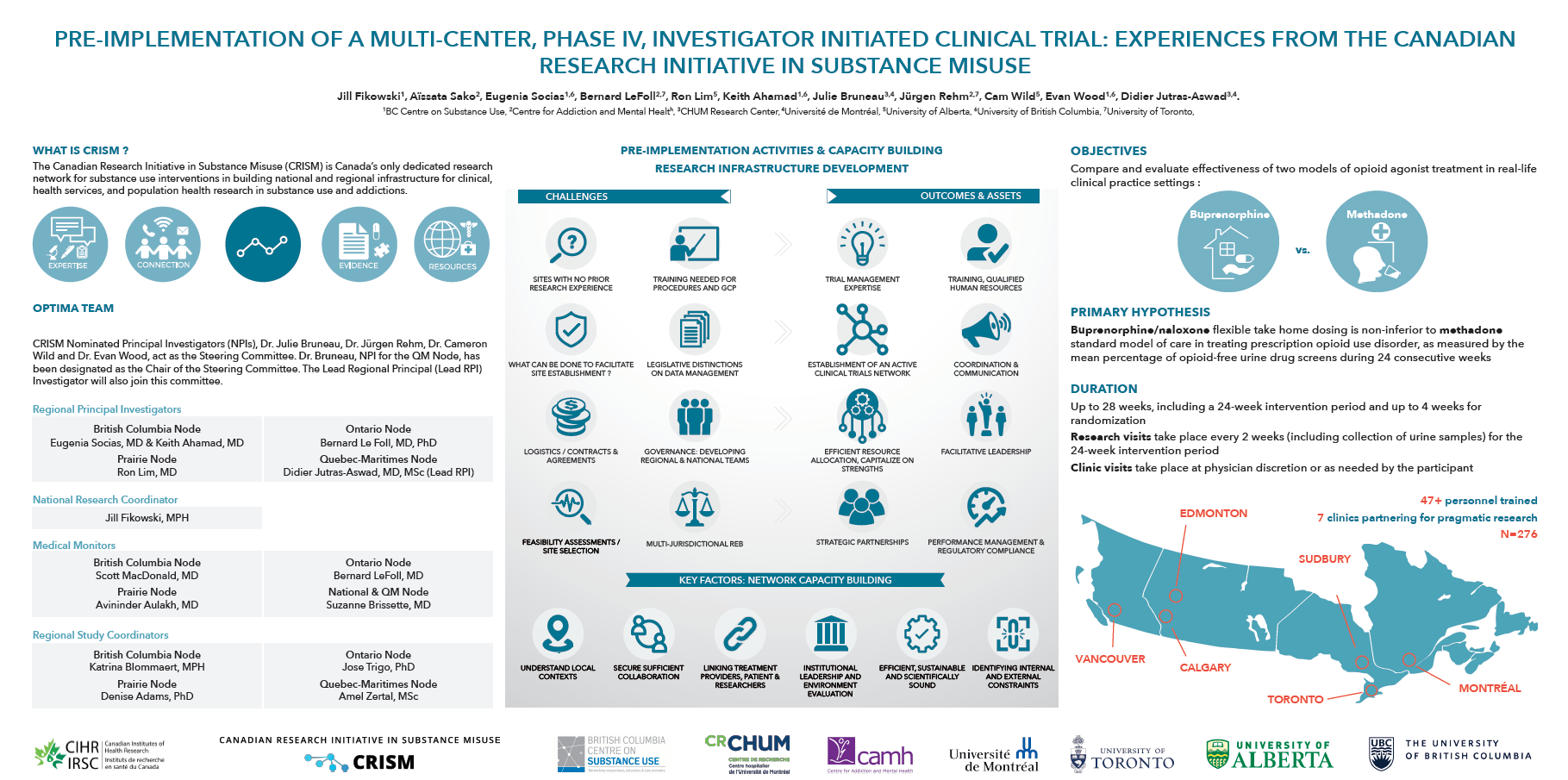
Although methadone has long been the standard of care for the treatment of opioid use disorder in Canada, there is growing consensus that the superior safety profile of buprenorphine/naloxone, as well as other comparative advantages, supports its use as a first-line therapy for opioid use disorder. The OPTIMA trial aims to evaluate these two treatment options within a Canadian practice‐based framework, generating evidence that is directly relevant to a recognized national priority in public health.
Objectives
Evaluate and compare the effectiveness of two different models of care for the treatment of prescription opioid use disorders (methadone and buprenorphine/naloxone) to improve patient care.
In Quebec
Steering committee
 Cameron Wild, PhD Prairies NPI
Cameron Wild, PhD Prairies NPI Evan Wood, MD, PhD British Columbia NPI
Evan Wood, MD, PhD British Columbia NPI Julie Bruneau, MD, MSc Quebec-Atlantic NPI
Julie Bruneau, MD, MSc Quebec-Atlantic NPI Jurgen Rehm, PhD Ontario NPI
Jurgen Rehm, PhD Ontario NPI
Regional principal investigators
 Bernard Le Foll, MD, PhD Ontario Node
Bernard Le Foll, MD, PhD Ontario Node Eugenia Socias, MD British Columbia Node
Eugenia Socias, MD British Columbia Node Keith Ahamad, MD British Columbia Node
Keith Ahamad, MD British Columbia Node Ronald Lim, MD Prairies Node
Ronald Lim, MD Prairies Node
National Team
 Aïssata Sako Program Director
Aïssata Sako Program Director Jill Fikowski, MPH National Research Coordinator
Jill Fikowski, MPH National Research Coordinator
Clinical research coordinators
 Amel Zertal, MSc Clinical Research Coordinator - Quebec-Atlantic Node
Amel Zertal, MSc Clinical Research Coordinator - Quebec-Atlantic Node Angela Wallace Clinical Research Coordinator - Prairies Node
Angela Wallace Clinical Research Coordinator - Prairies Node Benita Okocha, MSc Clinical research coordinator - BC node
Benita Okocha, MSc Clinical research coordinator - BC node Leanne Trick, MSc, PhD Clinical Research Coordinator - Ontario Node
Leanne Trick, MSc, PhD Clinical Research Coordinator - Ontario Node